* Simplified implantation through reduced suture steps.
For professional use. See instructions for use for full prescribing information, including
indications, contraindications, warnings, precautions, and adverse events.
Edwards Lifesciences devices placed on the European market meeting the essential requirements
referred to in Article 3 of theMedical Device Directive 93/42/ECC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, EDWARDS INTUITY and EDWARDS INTUITY Elite
are trademarks of Edwards Lifesciences Corporation.
© 2016 Edwards Lifesciences Corporation. All rights reserved. E6084/04-16/HVT
Edwards Lifesciences •
Route de l’Etraz 70, 1260 Nyon, Switzerland •
edwards.comEDWARDS
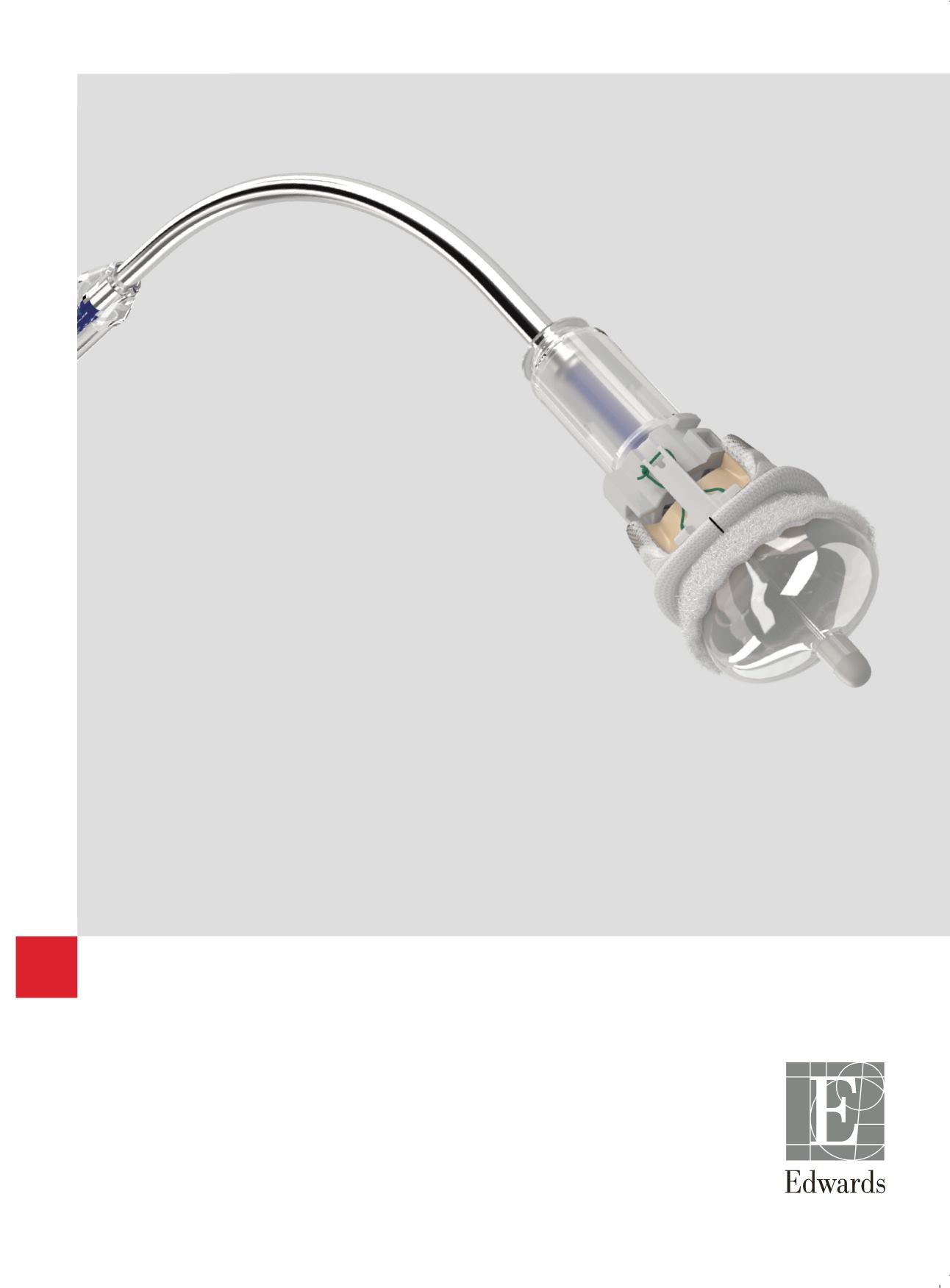
INTUITY Elite
Valve System
Trusted Platform
Rapid Deployment*
Smaller Incisions