
345
[CLINICAL RELEVANCE OF MOLECULAR MARKERS IN GLIOMAS - Varun Monga, MBBS, et al]
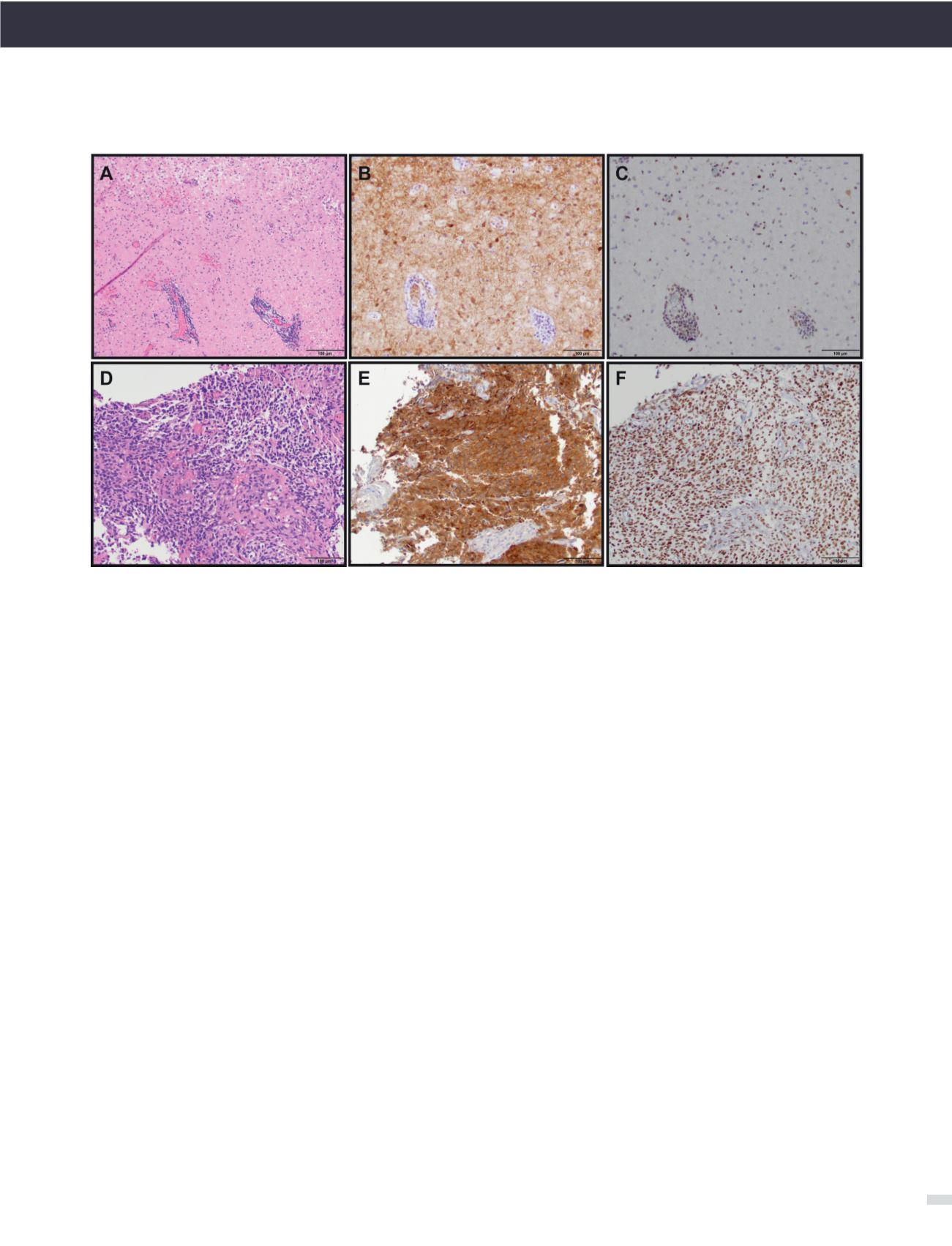
FIGURE 1. THE USE OF MOLECULAR MARKER IMMUNOHISTOCHEMISTRY TO DIAGNOSE GLIOMAS
(A) Diffuse astrocytoma (gemistocytic variant), WHO grade II (H&E, 200X original magnification). (B) IDH1 R132H immunostain showing tumor cell posi-
tivity (IDH1 R132H, 200X original magnification). (C) ATRX immunostain showing endothelial cell and lymphocyte positivity as internal positive controls
with tumor cells being negative (ATRX, 200X original magnification). Overall, the IDH and ATRX immunostains confirm the integrated diagnosis for
images from A-C of diffuse astrocytoma, IDH-mutant with a likely concomitant ATRX inactivating mutation. (D) Anaplastic oligodendroglioma, WHO
grade III with vascular proliferation (H&E, 200X original magnification). (E) IDH1 R132H immunostain showing tumor cell positivity (IDH1 R132H, 200X
original magnification). (F) ATRX immunostain showing diffuse tumor cell positivity along with endothelial cell positivity as an internal positive control.
In contrast to the astrocytoma, this oligodendroglioma did not show immunohistochemical evidence of an ATRX mutation; however, 1p/19q status by
FISH confirmed the presence of 1p/19q-codeletion (image not shown). Therefore, the integrated diagnosis for images from D-F is anaplastic oligoden-
droglioma, IDH-mutant, 1p/19q-codeleted.
impairing differentiation has been hypothesized (11,12).
Retrospective analyses of tissues from multiple clinical trials
have indicated
IDH
mutation to be a favorable prognostic
marker in adult low and high grade gliomas (10,13,14). The
long-term data of RTOG 9802 trial3, which included high risk
grade II glioma patients, reported outcomes stratified by
IDH
molecular status. Patients with tumoral
IDH1
R132H muta-
tions had significantly longer progression-free survival than
did those without the mutation (p=0.003) and among those
with the
IDH1
R132H mutation, patients receiving procar-
bazine, CCNU and vincristine plus radiation did better than
those receiving radiation therapy alone. EORTC 22033 also
released their short-term follow up data in similar high risk
grade II glioma patients (15). This is an open label, random-
ized, phase III study to receive conformal radiation therapy
versus dose-dense temozolomide 75mg/m
2
daily for 21 out
of 28 days repeated for 12 cycles. The patients were stratified
by
IDH
and 1p/19q-codeletion status prior to randomization,
making this a first-of-its-kind prospective study utilizing
molecular markers in low-grade glioma. The results showed
an improvement in progression-free survival (PFS) in patients
with
IDH
-mutant and 1p/19q-intact tumors treated with
radiation therapy than those treated with temozolomide and
no differences in PFS in the
IDH
-mutant/1p/19q-codeleted
and
IDH
-wildtype tumors. Median overall survival was not
reached, and future data may help understand the predic-
tive effect on different molecular subtypes of the two treat-
ments. An orally available inhibitor of both
IDH1/2
mutations
is currently being tested in clinical trials in gliomas, acute
myeloid leukemias and other solid tumors such as chon-
drosarcomas, and cholangiocarcinomas (NCT02746081,
NCT02632708, NCT02987010 and NCT02577406). Inhibi-
tion of this enzyme is proposed to block the production of
2-hydroxyglutarate (2-HG) thereby impairing proliferation
and promoting differentiation. Another potential target
being explored is inhibition of glutamine synthesis, which in
turn inhibits the conversion of glutamine to alpha keto gluta-
rate (16). Non-invasive metabolic imaging such as magnetic
resonance (MR) spectroscopy can detect 2- hydroxy glutarate
(HG) oncometabolite in tumors and help monitor treatment
response (17), but in some early studies such imaging has
shown to be associated with false-negative results (18,19).
















