616
MDR bacteria. Antimicrobial Stewardship Programs (ASPs)
have become widely popular in the United States and
Europe to address this unmet need (4). Such programs
aim to manage antimicrobial use in the acute care setting
through coordinated interventions designed to improve
and measure appropriate use. ASPs, therefore, promote
the selection of optimal antibiotic drug regimens including
dosing, duration of therapy, and route of administration
across the medical center. One component of ASPs is the
consideration and implementation of antibiotic regimens
based on pharmacodynamic concepts. Although the use of
pharmacodynamics to design antibiotic dosing regimens,
such as the continuous infusion of beta-lactams, has been
widely reported in the literature, the strategic design and
implementation of such programs as part of an ASP has been
more elusive.
Herein, a brief review of antimicrobial pharmacodynamics
is provided, followed by discussion of considerations and
strategy regarding where implementation of these dosing
strategies might provide the greatest benefits.
PHARMACODYNAMICS: WHAT’S THE RIGHT DOSE?
Inappropriate antibiotic therapy is most often a result
of delayed administration (i.e., waiting for culture or
susceptibility results before initiating antibiotics or
starting therapy as a result of a positive culture) or,
more often, an underestimation of current trends in
resistance. Regardless, the classification of an organism as
“Susceptible”, “Intermediate”, or “Resistant” does not inform
the prescriber of the ideal dose to use for the infection.
Instead, the term “optimal antibiotic therapy” should be
used and is meant to indicate that not only is the correct
antibiotic selected, but also that the dosage is sufficient
to obtain the maximal exposure threshold determined
from pharmacodynamic studies. An interesting observation
relevant to optimal antibiotic therapy is that the pathogen
need not be “Susceptible” to the drug in question, as long as
the exposure of the agent is sufficient to kill that organism.
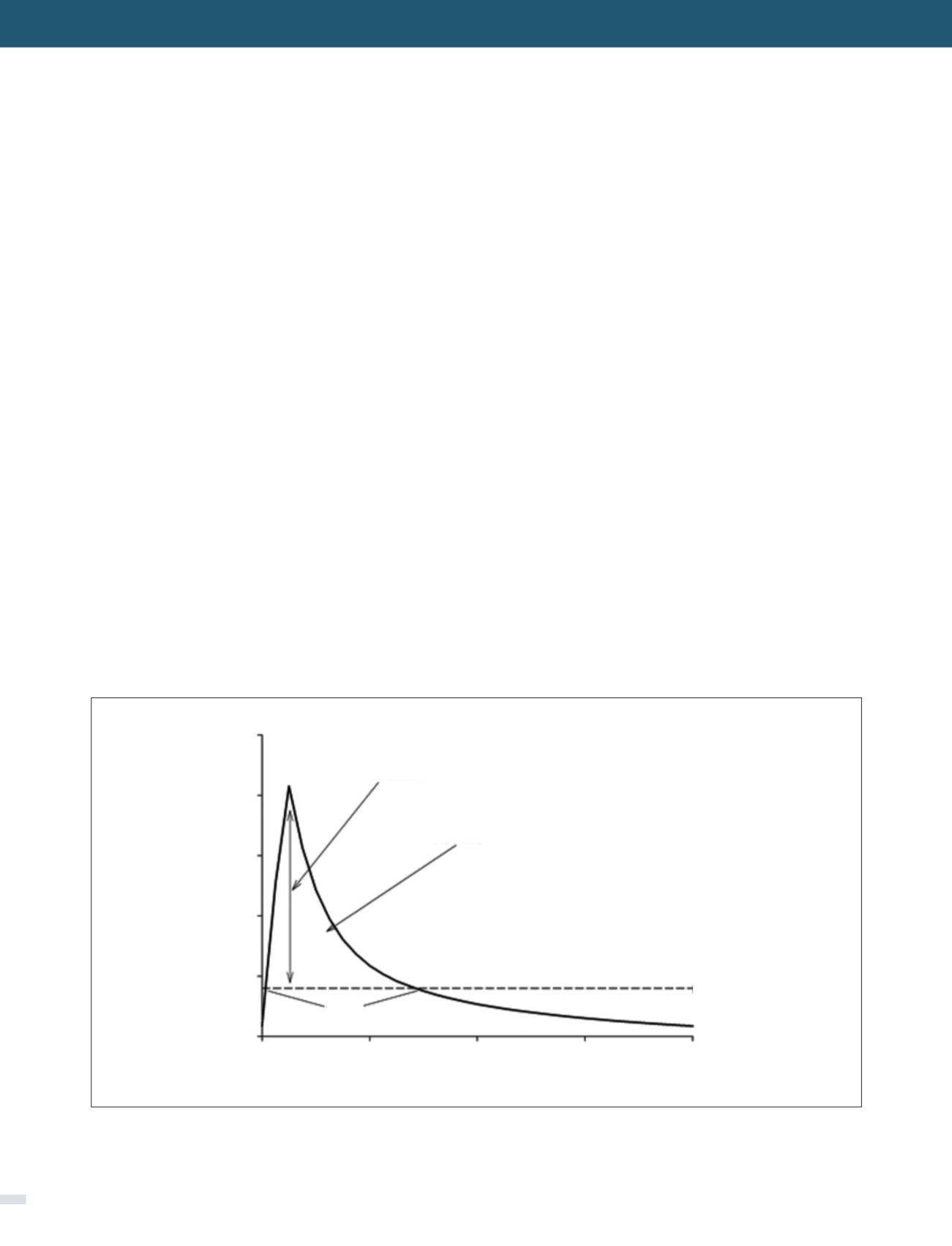
Antimicrobial killing characteristics
are dependent on
both the concentration of drug in relation to the
minimum
inhibitory concentration (MIC)
and
the time that
this exposure is maintained
(Figure 1) (5). When the
effect of concentration predominates over that of time,
the antibiotic displays concentration-dependent effects
that are significantly associated with an optimal free drug
maximum concentration to MIC ratio (fC
max
/MIC). When
the effect of time is greater, the antibiotic displays time-
dependent effects, and bacterial outcomes are associated
with free drug concentrations remaining above the MIC for a
FIGURE 1. DEPICTION OF PHARMACODYNAMIC PARAMETERS OVER A CONCENTRATION TIME PROFILE
MIC: Minimum inhibitory concentration; Cmax/MIC: Maximum concentration to MIC ratio; AUC/MIC: Area under the curve to MIC ratio; T>MIC: Time
above the MIC.
Concentration (mg/L)
Time (hr)
Cmax/MIC
AUC/MIC
MIC
T>MIC
50
40
30
20
10
0
0
2
4
6
8
[REV. MED. CLIN. CONDES - 2016; 27(5) 615-624]








